Treatment for 200 million patients of stress urinary incontinence
A new cell therapy for regenerating skeletal muscle with a person’s own stem cells could become a life-changer for millions of patients suffering from diseases such as muscle degeneration. The technology is being developed in Zurich.
Worldwide, around 200 million people are diagnosed with stress urinary incontinence, with women making up 75% of patients. Current therapeutic treatments focus on behavioural change such as avoiding caffeinated drinks or losing weight, as well as exercises to strengthen the pelvic floor and, in some situations, surgical interventions that are often limited in their efficacy.
Personalised cell therapy to regenerate skeletal muscle tissue
MUVON Therapeutics, a spin-off from the University of Zurich, is developing a novel, long-term solution by targeting the underlying cause of the disease using autologous cell therapy for the regeneration of the skeletal muscle tissue that supports bladder control. In other words, the therapeutic intervention uses an individual's cells, which are cultured and expanded outside the body, and then reintroduced into the patient. To date, personalised cell therapies have mostly been developed for deadly diseases. The Swiss spin-off wants to change this paradigm and bring personalised cell therapy to patients with conditions that, while non-life threatening, are debilitating and significantly affect day-to-day life.
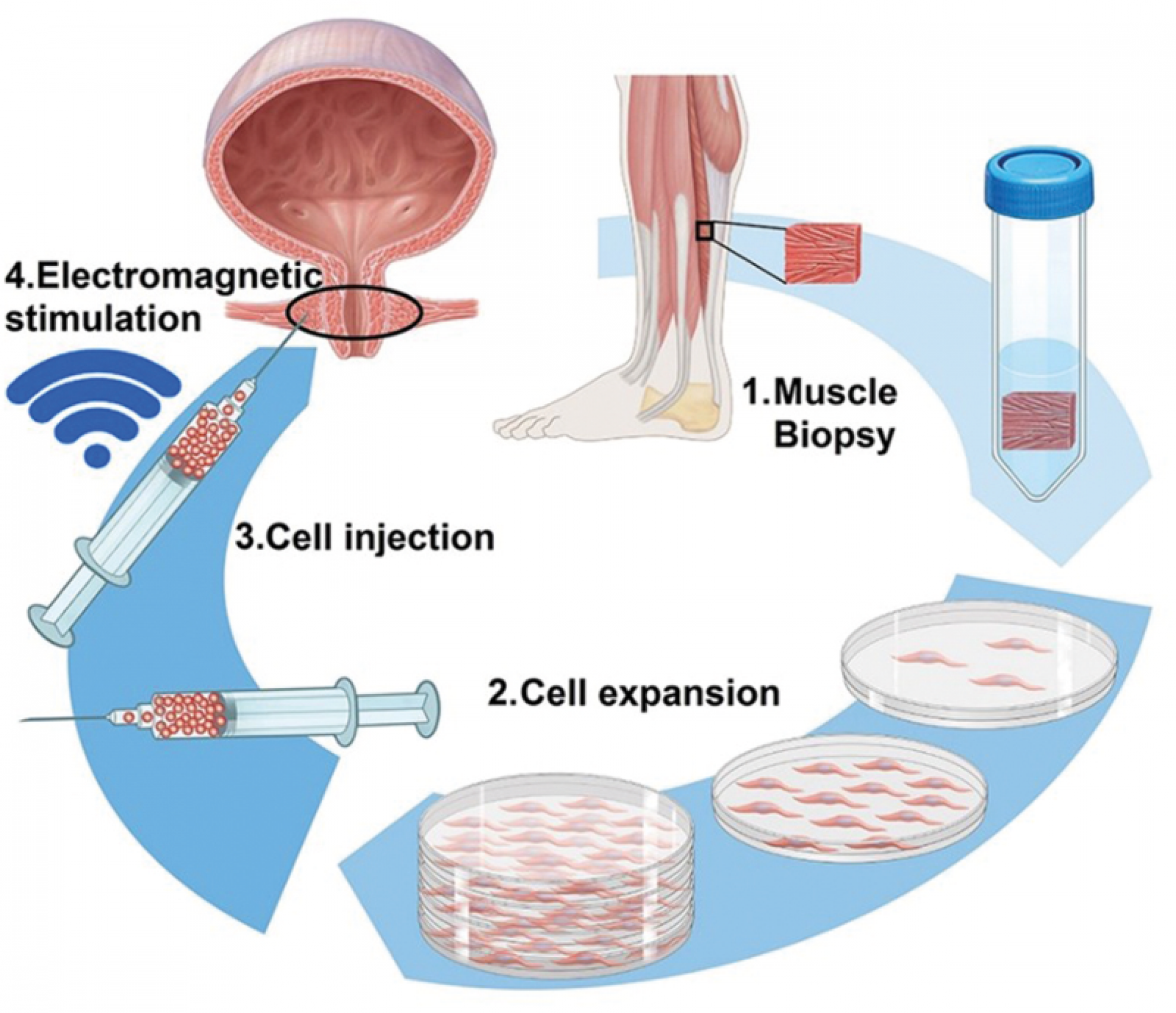
MUVON’s process for harvesting and re-injecting cells to stimulate muscle growth.
The new approach transplants a patient’s own muscle precursor cells from the lower leg into the sphincter of the bladder, followed by training of the pelvic floor. Once injected, the muscle precursor cells begin to differentiate, form new muscle fibres and connect with existing muscle tissue leading to regeneration of the muscle.
A decade of research
The research behind the technology started over a decade ago in the laboratory of Tissue Engineering and Regenerative Medicine of the University of Zurich. An international consortium led by the university was established with the goal of transferring the research results from lab to clinic. The project received funding from the European Union Horizon 2020 programme, leading to first in-human trials in January 2020 and successful completion in September 2021. To ensure that this promising therapy and technology is developed further and made available to the millions of patients in need, the research team decided to intensify efforts and to found MUVON Therapeutics as a spin-off.
With further support from Wyss Zurich, the required funds have been secured to start the second phase of clinical trials, focusing on evaluating the efficacy of the approach and undertaking all the required activities for regulatory approval.




